Incineration
Incineration is from the Latin incinerare, burning. The combustion process takes place in furnaces with a high oxygen content, which is necessary for the complete oxidation of the entire organic part. Products of gorenje these are ash and gas, as well as toxic substances, some with the effect of carcinogens. Generated heat it is usually used for generating electricity. In connection with the impact on human health and the high cost, this method is not a priority, but is actively used in countries where there is no large area for garbage storage.
Advantages of incineration
- The possibility of using combustion heat as an energy source
- The ability to recycle garbage without separation
- The possibility of placing the station near settlements
- Does not require a large area for processing
- Reduces the volume of solid waste by 80-85%
Disadvantages of incineration
- It does not completely remove all waste, so a special landfill is required to dispose of waste, many of which are very toxic
- Toxic gases are formed, which must be treated as dioxins (mutagenic and carcinogenic substances)
- The process technology requires an external energy source
- High processing cost and large investment
- Possibility of an accident
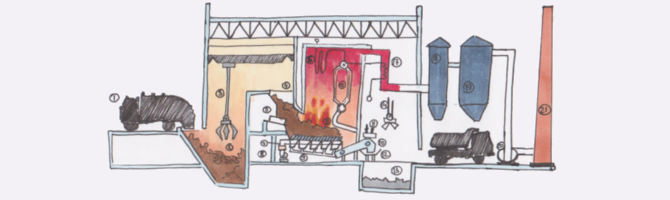
Principle of operation and device of the incinerator
First of all, the incinerator consists of a container in which waste is placed for subsequent incineration. Next is the furnace, which receives waste from the container and the required amount of oxygen. Ash in the furnace it goes down, and the gases exit into a separate chamber, where oxygen is added to completely burn the gas at a temperature of 1000°C for about 2-4 seconds, from which they exit through pollution level control after passing through a series of filters. The gases come out clean and with ambient temperature through the chimney, and the ash is transported by water to a special receiver for subsequent processing. Most incinerators a rotating furnace is used for better homogenization of waste. Combustion temperature depending on the process It ranges from 950°C to 1200°C.
Chemical processes in the incinerator
Under ideal conditions, using the required amount of oxygen O2 in stoichiometric proportions, the combustion products would consist of CO2, H2O, N2, SO2. The following chemical reactions would occur:
- C + O2 → CO2 + Heat
- 4H2 + O2 → 2H2O + Heat
- S +O2 → SO2
In practice, in addition to oxygen in the ground state O2, oxygen is required in all other states: RO, ROO, O, HO. Considering this fact and the fact that the combustion products can to react with each other, does not leave ideal conditions possible and as a result appears there are a lot of pollutants produced by the burning station.
Composition of incinerator waste
As a result of combustion, solid substances are formed in the form of ash, various gases, as well as liquid fractions and nothing from none of the above is of interest to the environment. The main components of garbage and their derivatives presented in the table below:
| Carbon | ash and carbon dioxide |
| Oxygen | carbon dioxide |
| Hydrogen | steam |
| Halogens | Acids |
| Nitrogen | Nitric oxide |
| Phosphorus | phosphorus oxide |
| Metals | metal oxides |
| Alkalines | hydroxides |
Nitrogen oxides NOx
The most important of them are NO and NO2, participate in the formation of ozone (O3 strong oxidizer, lung irritation), peroxyacetyl nitrate (eye irritation), photochemical smog, as well as acid rain and clouds.
Sulfur oxide (IV)
It is formed from waste containing sulfur, an irritant gas for the eyes, nose and throat. In high concentrations it can lead to death in people with respiratory diseases. The main source of acid rain.
Carbon monoxide
The product of incomplete combustion, when ingested, reacts with hemoglobin to form carboxyhemoglobin, replacing oxyhemoglobin, which supplies oxygen to the tissues. As a result, oxygen starvation occurs: headaches, shortness of breath and other problems are possible, with prolonged consumption in large quantities concentration leads to death.
Metals
Various kinds of metals can remain both in ash and in gases. In gases coming from incinerators The elements Cd, Zn, Sb, Ag, In and Sn, as well as Hg, were found in smaller quantities. The ability to evaporate or stay in the ash depends on the nature of the chemical element. So, metals are divided into three different groups:
- Al, Ba, Be, Ca, Co, Fe, K, Mg, Mn, Si, Sr and Ti - have high boiling points, so in the chamber incineration does not evaporate and remains in the ash
- As, Cd, Cu, Pb, Zn, Sb and Se - evaporate during combustion, but in the process of cooling the gas easily condense, therefore, as a rule, they remain in the ash
- Hg - evaporates and does not condense, therefore, as a rule, it comes out with exhaust gases
Due to the possible toxicity of metals and their compounds, the control of exhaust gases should be comprehensive.
Acid gases
The combustion of chlorine- and fluorinated waste leads to the formation of acid gases, such as hydrogen fluoride (hydrofluoric acid) and hydrogen chloride. Fluorine is found in many products, chlorine - in plastics, compounds such as polyvinyl chloride (PVC) can be contained in polystyrene (PS) and polyethylene (PE), which, as a rule, contain additives with chlorine.