Chemical compounds
After reading the article, you will learn how molecules are formed, what processes occur when atoms bond, and what there are types of connections
A chemical compound is a substance consisting of two or more atoms connected by a chemical bond. Organic chemical compounds are described in section organic chemistry.
Molecule
Different atoms can connect with each other and form complex compounds - molecules. An example of a molecule it can be H2O - water, HCl - hydrochloric acid or H2SO4 - sulfuric acid. When two atoms are at close distance, their electron clouds can overlap and form a compound - a molecule, while the nuclei of atoms will be located inside one electron cloud.
At a great distance, the atoms do not interact with each other, at some distance the atoms attract atoms repel each other and at very close distances. At some point, the forces of repulsion and attraction the atoms are balanced and can combine into a molecule. The emergence of communication is mainly influenced by electronic the configuration of each of the atoms. The fact is that each electron moves along a certain orbital, i.e. it has some level of energy. The formation of a bond between atoms means the creation of common electronic orbitals, i.e. other, new energy levels are being created, along which electrons move.
Molecular orbitals
Molecular orbitals are a method for determining the configuration of the electron shell of a molecule, as well as its properties. At each energy level of the molecular orbital, as well as on the atomic orbital, there are two electrons. In this case, the levels are formed so that the first strengthens the bond between the atoms, and the next one weakens (loosens) the bond. Figure 2 shows what σ- and π-connections look like in space. Figure 1 shows the redistribution of electrons from atomic orbitals to molecular orbitals. The sequence of fillings of molecular orbitals is as follows: σ1s2 < σ1s2* < σ2s2 < σ2s2* < σ2px2 < π2py2 = π2pz2 < π2py2* = π2pz2* < σ2px2* and so on. On the first molecular orbital, a σbond is formed - this is a simple bond that is directed in space along the line that connects the centers of atoms, then, at the first energy level, a destructive σ-link (marked with *) and so on. The strengthening bond 1σ has a lower energy level than the 1s orbital, loosening bond σ* it has a higher energy level than the 1s orbital, so this state unstable: electrons from the 1s orbital need to move to a less stable state. The situation is similar with higher-energy orbitals.
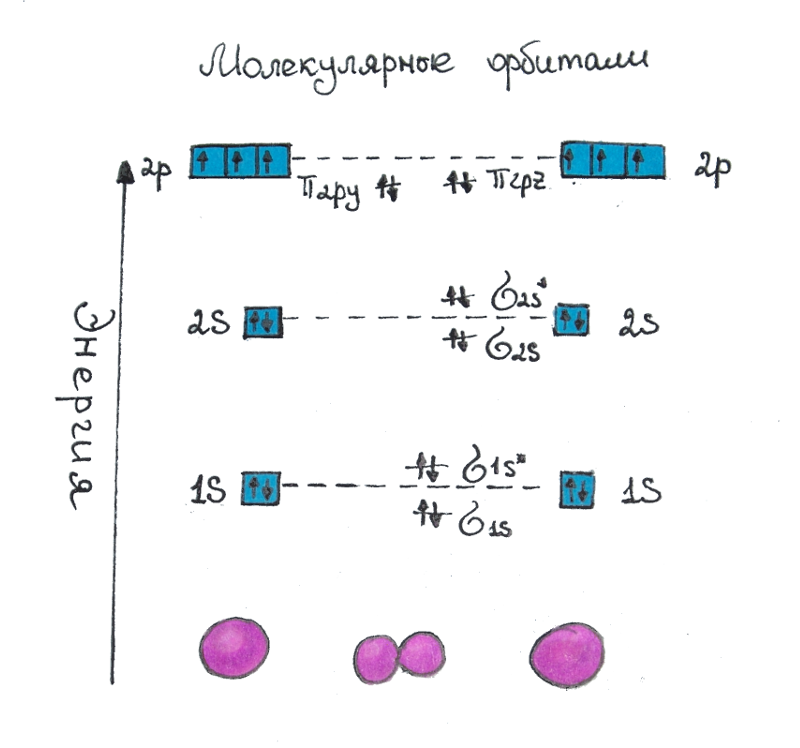 Fig.1 Molecular orbitals. The energy level of molecular orbitals relative to atomic orbitals. The arrangement of electrons in the molecule N2.
Fig.1 Molecular orbitals. The energy level of molecular orbitals relative to atomic orbitals. The arrangement of electrons in the molecule N2.
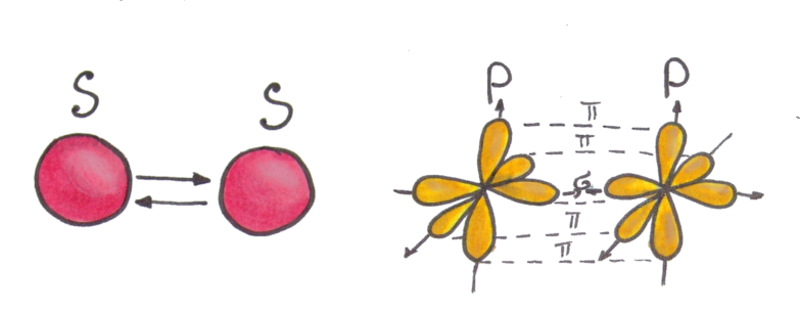 Fig.2 Sigma and pi bonds in a molecule between two atoms. When p-orbitals interact, two pi-bonds and one sigma-bond are formed
Fig.2 Sigma and pi bonds in a molecule between two atoms. When p-orbitals interact, two pi-bonds and one sigma-bond are formed
Magnetic properties of the substance
The magnetic properties of a substance are determined by its electronic configuration, regardless of whether it is an atom or a molecule. If one or more electrons in the shell are without a pair, then the substance formed by such molecules will be possess paramagnetic properties - will be magnetized in an external magnetic field. Otherwise, the substance it is called a diamagnet: in the presence of a magnetic field, it is magnetized against the direction of the field and in the absence of a field non-magnetic.
Ionic coupling
The most common types of bonds in a molecule are: covalent, metallic and ionic bonds. Ionic coupling it is based on the attraction of opposite charges - cations and anions. For example, sodium cation Na+ for One electron is needed to return to the ground state, the chlorine anion Cl- must be given one electron to return to the ground state. basic condition. Thus, when Na+ and Cl- interact, in the formed molecule they share one electron among themselves, which allows them to be in a stable state.