Beryllium Be
Beryllium is the 4 element in periodic table situated in 2 period.| Symbol | Be |
| Number | 4 |
| Atomic weight | 9.0121831 |
| Latin name | Beryllium |
| English name | Beryllium |
Electronic configuration of of Beryllium
Be: 1s2 2s2
Short notation:
Be: [He]2s2
Same electronic configuration has an atom of Beryllium and Li-1, B+1, C+2, N+3
The order of filling the shells with electrons of Beryllium (Be): 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
On the sub level ‘s’ there might be 2 electrons at most, on ‘p’ - up to 6, on ‘d’ - up to 10 and up to 14 on ‘f’
Beryllium has 4 electrons, let's fill electronic layers in described order:
2 electrons on 1s-sub level
2 electrons on 2s-sub level
Oxidation state of Beryllium
Atoms of Beryllium in compounds have an oxidation state of 2.
The oxidation state is the conditional charge of an atom in a compound: the bond in a molecule between atoms is based on the sharing of electrons, thus, if the atom’s charge virtually increases, then the oxidation state is negative (electrons carry a negative charge), if the charge decreases, then the oxidation state is positive.
Ions of Beryllium
Valence of Be
Atoms of Beryllium in compounds have valence II.
Valence of Beryllium is an ability of an atom Be to build chemical bounds. The valence is based on electronic configuration of atom: electrons participated in chemical bounds are known as valence electrons. In general the valence is:
The number of possible chemical bounds with other atoms
The valence has no sign.
Quantum numbers Be
Quantum numbers are defined by the last electron in configuration, for an atom Be these numbers are N = 2, L = 0, Ml = 1, Ms = -½
Filling an electronic configuration (gif):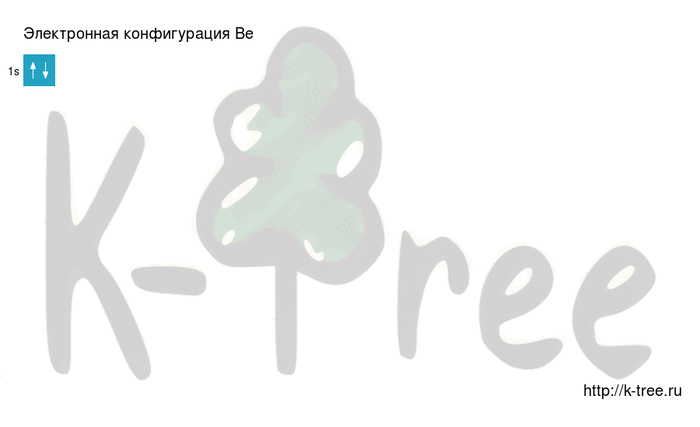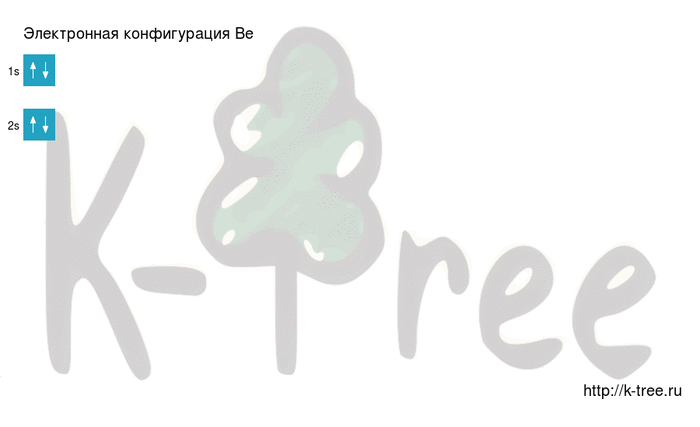
Result:

Compounds of Beryllium
| Type | Formula | Name |
|---|---|---|
| Salt | BeHPO4 | Beryllium hydroorthophosphate |
| Be(H2PO4)2 | Beryllium Dihydroorthophosphate | |
| Base | Be(OH)2 | Beryllium hydroxide |
| Oxide | BeO | Beryllium oxide |
Ionization energy
The closer the electron is to the center of the atom, the more energy is needed to tear it off. The energy spent on removing an electron from an atom is called ionization energy and is designated Eo. Unless otherwise stated, the ionization energy is the energy of removal of the first electron, and there are also ionization energies for each subsequent electron.
Ionization energy of Be:
Eo = 900 kJ/mol
See all elements of the periodic table
Where is Be in the periodic table?