Hafnium Hf
Hafnium is the 72 element in periodic table situated in 6 period.| Symbol | Hf |
| Number | 72 |
| Atomic weight | 178.4900000 |
| Latin name | Hafnium |
| English name | Hafnium |
Electronic configuration of of Hafnium
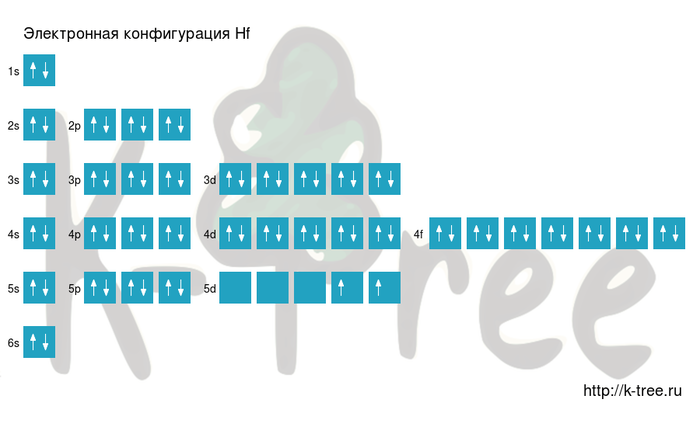
Hf: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d2
Short notation:
Hf: [Xe]6s2 4f14 5d2
Same electronic configuration has an atom of Hafnium and Ta+1, W+2, Re+3, Os+4, Ir+5
The order of filling the shells with electrons of Hafnium (Hf): 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
On the sub level ‘s’ there might be 2 electrons at most, on ‘p’ - up to 6, on ‘d’ - up to 10 and up to 14 on ‘f’
Hafnium has 72 electrons, let's fill electronic layers in described order:
2 electrons on 1s-sub level
2 electrons on 2s-sub level
6 electrons on 2p-sub level
2 electrons on 3s-sub level
6 electrons on 3p-sub level
2 electrons on 4s-sub level
10 electrons on 3d-sub level
6 electrons on 4p-sub level
2 electrons on 5s-sub level
10 electrons on 4d-sub level
6 electrons on 5p-sub level
2 electrons on 6s-sub level
14 electrons on 4f-sub level
2 electrons on 5d-sub level
Oxidation state of Hafnium
Atoms of Hafnium in compounds have an oxidation state of 4, 3, 2, 1.
The oxidation state is the conditional charge of an atom in a compound: the bond in a molecule between atoms is based on the sharing of electrons, thus, if the atom’s charge virtually increases, then the oxidation state is negative (electrons carry a negative charge), if the charge decreases, then the oxidation state is positive.
Ions of Hafnium
Valence of Hf
Atoms of Hafnium in compounds have valence IV, III, II, I.
Valence of Hafnium is an ability of an atom Hf to build chemical bounds. The valence is based on electronic configuration of atom: electrons participated in chemical bounds are known as valence electrons. In general the valence is:
The number of possible chemical bounds with other atoms
The valence has no sign.
Quantum numbers Hf
Quantum numbers are defined by the last electron in configuration, for an atom Hf these numbers are N = 5, L = 2, Ml = -1, Ms = +½
Filling an electronic configuration (gif):
Result:
Ionization energy
The closer the electron is to the center of the atom, the more energy is needed to tear it off. The energy spent on removing an electron from an atom is called ionization energy and is designated Eo. Unless otherwise stated, the ionization energy is the energy of removal of the first electron, and there are also ionization energies for each subsequent electron.
Ionization energy of Hf:
Eo = 659 kJ/mol
See all elements of the periodic table
Where is Hf in the periodic table?