Ion Br- (Minus one)
| Symbol | Br- |
| Number | 35 |
| Atomic weight | 79.9010000 |
| Latin name | Bromum |
| English name | Bromine |
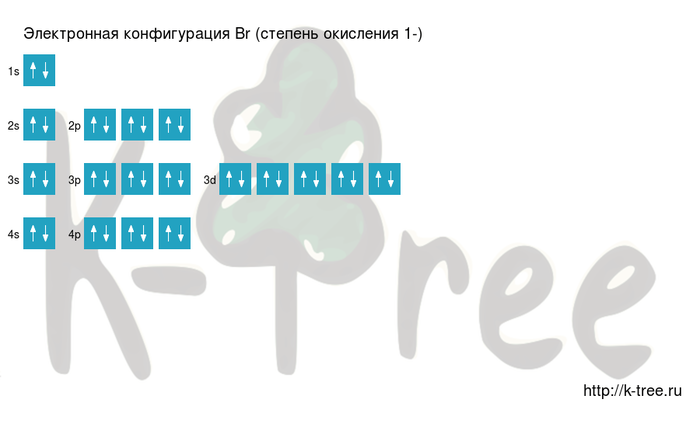
Electronic configuration of of Bromine
Br: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 → Br-:1s22s22p63s23p64s23d104p6
Same electronic configuration has an ion of Bromine -1 and As-3, Se-2, Kr, Rb+1, Sr+2, Y+3, Zr+4, Tc+7
The order of filling the shells with electrons of Bromine (Br-): 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
On the sub level ‘s’ there might be 2 electrons at most, on ‘p’ - up to 6, on ‘d’ - up to 10 and up to 14 on ‘f’
Bromine has 35 electrons, let's fill electronic layers in described order:
2 electrons on 1s-sub level
2 electrons on 2s-sub level
6 electrons on 2p-sub level
2 electrons on 3s-sub level
6 electrons on 3p-sub level
2 electrons on 4s-sub level
10 electrons on 3d-sub level
6 electrons on 4p-sub level
Oxidation state of Bromine
Atoms of Bromine in compounds have an oxidation state of 7, 5, 4, 3, 1, 0, -1.
The oxidation state is the conditional charge of an atom in a compound: the bond in a molecule between atoms is based on the sharing of electrons, thus, if the atom’s charge virtually increases, then the oxidation state is negative (electrons carry a negative charge), if the charge decreases, then the oxidation state is positive.
Oxidation state of an ion Br- = -1
Ions of Bromine
Valence of Br-
Atoms of Bromine in compounds have valence VII, V, IV, III, I.
Valence of Bromine is an ability of an atom Br to build chemical bounds. The valence is based on electronic configuration of atom: electrons participated in chemical bounds are known as valence electrons. In general the valence is:
The number of possible chemical bounds with other atoms
The valence has no sign.
Quantum numbers Br 1-
Quantum numbers are defined by the last electron in configuration, for an ion Br these numbers are N = 4, L = 1, Ml = 1, Ms = -½
Filling an electronic configuration (gif):
Result:
See all elements of the periodic table
Where is Br in the periodic table?